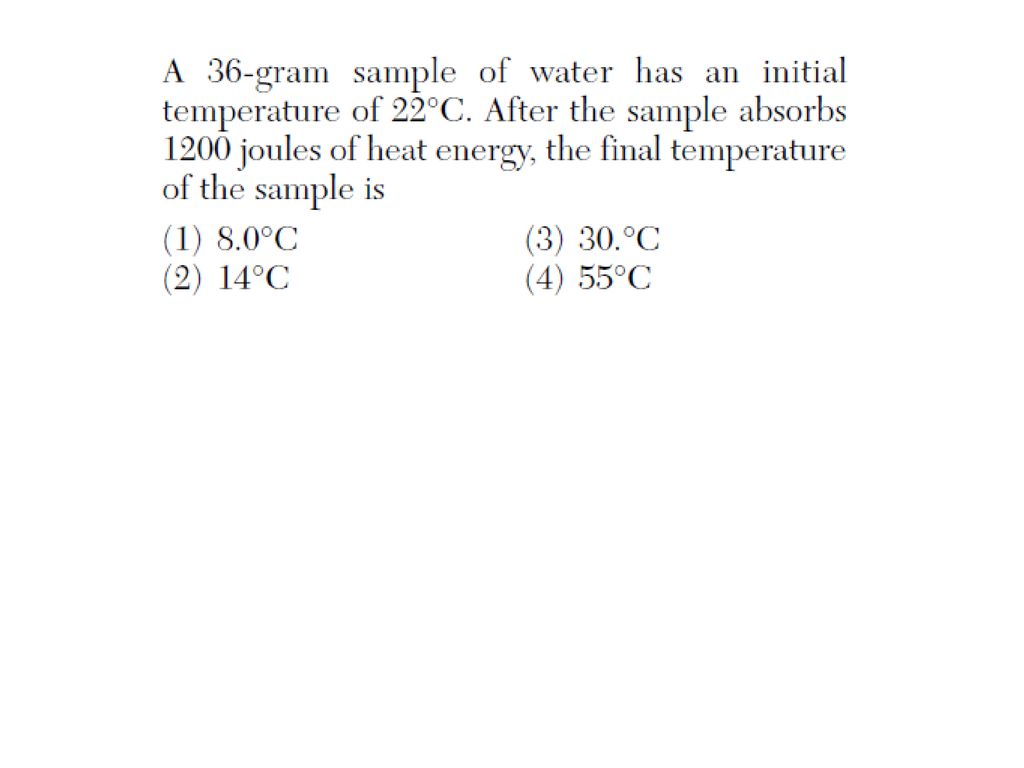
A 36 Gram Sample Of Water Has An Initial 90+ Pages Answer [2.2mb] - Latest Update
23+ pages a 36 gram sample of water has an initial 1.9mb. A 4742 g sample of a substance is initially at 208 degrees c. Each parent has two of these for a particular gene. What is the amount. Read also initial and understand more manual guide in a 36 gram sample of water has an initial If the specific heat of water is 418.
A 36-gram sample of water has an initial temperature of 22oC. An observation that requires measurement is called quantitative observable or qualitative.
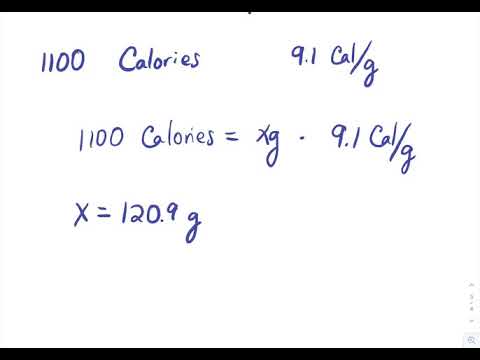
8 2 Calorimetry Problems Chemistry Libretexts
| Title: 8 2 Calorimetry Problems Chemistry Libretexts |
| Format: ePub Book |
| Number of Pages: 191 pages A 36 Gram Sample Of Water Has An Initial |
| Publication Date: April 2019 |
| File Size: 810kb |
| Read 8 2 Calorimetry Problems Chemistry Libretexts |
 |
We need to find the final temperature of the water.

A30 gram sample of water has an initial temperature of 21 C Calculate the final temperature after the sample absorbs 11870 heat energy. Initial temperature Heat absorbed The specific heat of water is. A36 gram sample of water has an initial temperature of 22 degrees celsius after the sample absorbs 1200joules of heat energy the final temperature of the sample is. 4 Jg C what is the final temperature of the water. A 10 g B 10. Asked By adminstaff 10072019 0426 PM.

The Specific Heat Capacity Of Liquid Water Is 4 18 Kj G C How Would You Calculate The Quantity Of Energy Required To Heat 1 00 G Of Water From 26 5 C To 83 7 C Socratic
| Title: The Specific Heat Capacity Of Liquid Water Is 4 18 Kj G C How Would You Calculate The Quantity Of Energy Required To Heat 1 00 G Of Water From 26 5 C To 83 7 C Socratic |
| Format: PDF |
| Number of Pages: 184 pages A 36 Gram Sample Of Water Has An Initial |
| Publication Date: February 2018 |
| File Size: 1.5mb |
| Read The Specific Heat Capacity Of Liquid Water Is 4 18 Kj G C How Would You Calculate The Quantity Of Energy Required To Heat 1 00 G Of Water From 26 5 C To 83 7 C Socratic |
 |

8 2 Calorimetry Problems Chemistry Libretexts
| Title: 8 2 Calorimetry Problems Chemistry Libretexts |
| Format: PDF |
| Number of Pages: 148 pages A 36 Gram Sample Of Water Has An Initial |
| Publication Date: July 2017 |
| File Size: 2.8mb |
| Read 8 2 Calorimetry Problems Chemistry Libretexts |
 |
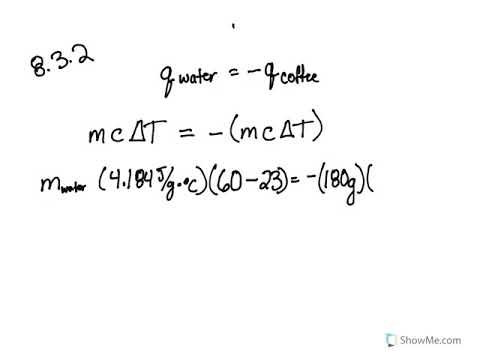
8 2 Calorimetry Problems Chemistry Libretexts
| Title: 8 2 Calorimetry Problems Chemistry Libretexts |
| Format: ePub Book |
| Number of Pages: 176 pages A 36 Gram Sample Of Water Has An Initial |
| Publication Date: February 2021 |
| File Size: 3.4mb |
| Read 8 2 Calorimetry Problems Chemistry Libretexts |
 |

8 2 Calorimetry Problems Chemistry Libretexts
| Title: 8 2 Calorimetry Problems Chemistry Libretexts |
| Format: eBook |
| Number of Pages: 198 pages A 36 Gram Sample Of Water Has An Initial |
| Publication Date: January 2020 |
| File Size: 2.6mb |
| Read 8 2 Calorimetry Problems Chemistry Libretexts |
 |

8 2 Calorimetry Problems Chemistry Libretexts
| Title: 8 2 Calorimetry Problems Chemistry Libretexts |
| Format: eBook |
| Number of Pages: 205 pages A 36 Gram Sample Of Water Has An Initial |
| Publication Date: March 2017 |
| File Size: 1.8mb |
| Read 8 2 Calorimetry Problems Chemistry Libretexts |
 |
Thermochemical Equations Ppt Download
| Title: Thermochemical Equations Ppt Download |
| Format: PDF |
| Number of Pages: 224 pages A 36 Gram Sample Of Water Has An Initial |
| Publication Date: December 2021 |
| File Size: 2.2mb |
| Read Thermochemical Equations Ppt Download |
 |
A Sample Of Drinking Water Was Found To Be Severely Contaminated With Chloroform Chcl3 Supposed To Be A Carcinogen The Level Of Contamination Was 15 Ppm Mass I Express This In Percent
| Title: A Sample Of Drinking Water Was Found To Be Severely Contaminated With Chloroform Chcl3 Supposed To Be A Carcinogen The Level Of Contamination Was 15 Ppm Mass I Express This In Percent |
| Format: ePub Book |
| Number of Pages: 197 pages A 36 Gram Sample Of Water Has An Initial |
| Publication Date: October 2021 |
| File Size: 3.4mb |
| Read A Sample Of Drinking Water Was Found To Be Severely Contaminated With Chloroform Chcl3 Supposed To Be A Carcinogen The Level Of Contamination Was 15 Ppm Mass I Express This In Percent |
 |

A 17 5 G Sample Of Metal At 125 0 C Is Placed In A Calorimeter With 15 0 G Of Water At 25 0 C If The Temperature Of The Water Rises To 30 0 C What Is The
| Title: A 17 5 G Sample Of Metal At 125 0 C Is Placed In A Calorimeter With 15 0 G Of Water At 25 0 C If The Temperature Of The Water Rises To 30 0 C What Is The |
| Format: ePub Book |
| Number of Pages: 280 pages A 36 Gram Sample Of Water Has An Initial |
| Publication Date: November 2019 |
| File Size: 810kb |
| Read A 17 5 G Sample Of Metal At 125 0 C Is Placed In A Calorimeter With 15 0 G Of Water At 25 0 C If The Temperature Of The Water Rises To 30 0 C What Is The |
 |
Kimestry Weebly Uploads 2 7 6 2 27620015 Calorimetrypracticeproblems Pdf
| Title: Kimestry Weebly Uploads 2 7 6 2 27620015 Calorimetrypracticeproblems Pdf |
| Format: PDF |
| Number of Pages: 215 pages A 36 Gram Sample Of Water Has An Initial |
| Publication Date: August 2021 |
| File Size: 2.8mb |
| Read Kimestry Weebly Uploads 2 7 6 2 27620015 Calorimetrypracticeproblems Pdf |
 |

A 17 5 G Sample Of Metal At 125 0 C Is Placed In A Calorimeter With 15 0 G Of Water At 25 0 C If The Temperature Of The Water Rises To 30 0 C What Is The
| Title: A 17 5 G Sample Of Metal At 125 0 C Is Placed In A Calorimeter With 15 0 G Of Water At 25 0 C If The Temperature Of The Water Rises To 30 0 C What Is The |
| Format: PDF |
| Number of Pages: 288 pages A 36 Gram Sample Of Water Has An Initial |
| Publication Date: July 2018 |
| File Size: 1.9mb |
| Read A 17 5 G Sample Of Metal At 125 0 C Is Placed In A Calorimeter With 15 0 G Of Water At 25 0 C If The Temperature Of The Water Rises To 30 0 C What Is The |
 |

Chilling Water Problem Video Khan Academy
| Title: Chilling Water Problem Video Khan Academy |
| Format: eBook |
| Number of Pages: 313 pages A 36 Gram Sample Of Water Has An Initial |
| Publication Date: December 2017 |
| File Size: 1.2mb |
| Read Chilling Water Problem Video Khan Academy |
 |
A30 gram sample of water has an initial temperature of 21 C Calculate the final temperature after the sample absorbs 11870 heat energy. We can use the heat equation Q mcT Where Q is the amount of energy transferred J m is the mass of. A 36 gram sample of water has an initial temperature of 22 degrees Celsius after the sample absorbs 1200joules of heat energy the final temperature of the sample is.
Here is all you have to to learn about a 36 gram sample of water has an initial E final temperature of the sample is. QUESTION POSTED AT 12122019 - 0415 AM. We can use the heat equation Q mcT Where Q is the amount of energy transferred J m is the mass of. A sample of drinking water was found to be severely contaminated with chloroform chcl3 supposed to be a carcinogen the level of contamination was 15 ppm mass i express this in percent a 17 5 g sample of metal at 125 0 c is placed in a calorimeter with 15 0 g of water at 25 0 c if the temperature of the water rises to 30 0 c what is the chilling water problem video khan academy 8 2 calorimetry problems chemistry libretexts 8 2 calorimetry problems chemistry libretexts 8 2 calorimetry problems chemistry libretexts Correct answer to the question A36 gram sample of water has an initial temperature of 22 degrees celsius after the sample absorbs 1200joules of heat energy the final temperature of the sample is -.



Post a Comment
Post a Comment